Learn
Gas Characteristics
As we learned earlier in this course, there are certain properties of the gas state of matter. Many elements and compounds are gas at room temperature. Refresh your memory about the phases of matter by watching the video The 3 Phases | Phases of Matter (1:20). PBS login information.
Let's discuss some key properties of the gas state of matter in detail:
Volume
The volume of a gas is simply the amount of space that the gas takes up. Gases tend to expand to fill their containers. Volume of gas is often measured in liters (L) or milliliters (mL).

Temperature
Temperature is often described as a measure of how hot or cold a substance is. The same is true for gases. Temperature is measured in degrees Celsius (°C).
Pressure
The pressure of a gas is the force a gas exerts per unit of area. For a gas in a container, this could also be stated as the force the gas puts on the walls of its container since the particles in the gas are constantly bouncing off the sides.
A common example of pressure is atmospheric pressure, which presses down a value of roughly 14.6959 psi (pounds per square inch)! You are feeling that pressure right now on every inch of your body but we are used to living under the pressure.
Confined Gas
"Confined gas" is simply a description used for a gas that cannot escape its container. A balloon is an example of a confined gas.

Density
Do you remember this term from earlier in the course? Density is mass per volume. The closer together gas molecules are to each other, the more densely packed they are.
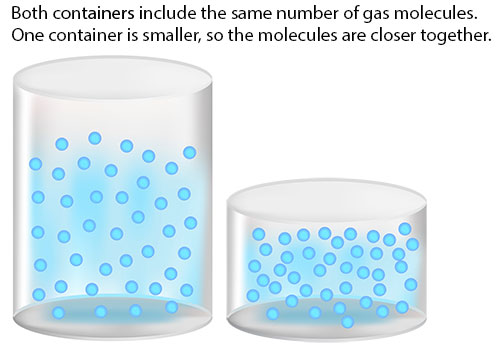
Lab Overview
Now that you are familiar with gases and their characteristics, you will be conducting some investigations to see the relationships that exist between the volume, pressure, and temperature of a confined gas.
You will be conducting 2 investigations:
- You will be squeezing a balloon to demonstrate how pressure affects volume
- You will be placing 3 balloons of roughly the same size in 3 different locations with different temperatures, leaving them for an hour, and comparing their sizes after an hour.
Review the next tab to see what materials you will need.
For these investigations, you will not be taking quantitative measurements measurements that are quantifiable, such as weighing the mass of an inflated balloon in grams or calculating the volume of a liquid in milliliters . Instead of taking measurements, you will record detailed observations of what you witness during the investigations and then use those observations to draw some conclusions. Detailed instructions are found in the 2.04 Gas Investigations Lab. You will be taking pictures of each investigation and including them on your lab report.
When you take your observations, make sure you are detailed and descriptive about what you are seeing. This is good scientific practice and will also help you draw conclusions later on in the lab.
Lab Materials
For these investigations you will need the following:
- 4-6 balloons that will inflate to roughly the same size (You should only really need 4 but a few extra are handy if some pop!)
- Access to a freezer
- Access to a “hot place” such as outside if it is hot, a car parked outside, near a sunny window, etc.
- A camera to take pictures of you performing the investigations.
When you are ready, proceed to the Task page to complete your investigations. After you complete your investigations, you will be sharing your results with your classmates in a discussion so you can see and discuss what others saw in their investigations.

