Learn
pH
pH is a measure of the amount of hydronium ions present in a solution. Because scientists don't like to work with negative numbers, they have set the scale so that a lower pH indicates a higher acidity.
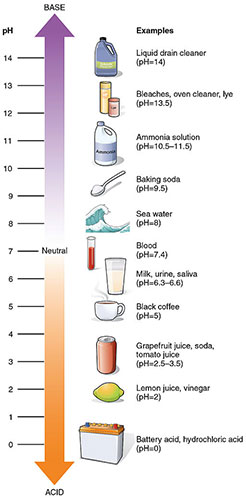
pH scale with examples. Image attribution: The pH scale (Figure 4) by OpenStaxCollege and Connexions is licensed under CC BY 3.0.
Take a look at the example pH scale above. See a larger version of the pH scale here. Notice the following:
- A pH of 7 is neutral, meaning the solution has equal concentrations of H3O+ ions and OH- ions. It is neither an acid nor a base.
- Acids are solutions with a pH lower than 7. The lower the pH value, the more acidic the solution.
- Bases have a higher a higher pH than 7. The higher the pH, the more basic the solution.
- Strong acids are usually considered to have a pH of 0-3, while weak acids have a pH of about 4-6.
- Strong bases are usually considered to have a pH of 12-14, while weak bases have a pH of 8-11.