Learn

Acids
Begin by watching the video Acids and Bases: The Voyage of the Proton and stop at the 13:03 minute mark.
An acid is a substance that produces hydrogen ions (H+) in a water solution. Acids' aqueous solutions are characterized by the following:
- Sour taste
- Corrosive: Able to damage or destroy materials like metals
- Turn blue litmus paper red
- React with bases and certain metals (like calcium) to form salts
While acids are characterized by a sour taste, never taste a substance to test for the presence of acids. Some acids can damage tissue by causing burns.
In an aqueous solution, the positive hydrogen ions (H+) dissolve in the water and then interact with the water molecules to form hydronium ions — (H3O+).
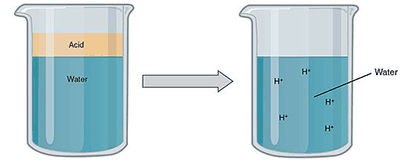
An acid dissolves into an aqueous solution. Image attribution: Acids and Bases (Figure 3) by OpenStaxCollege and Connexions is licensed under CC BY 3.0. Cropped from original.
Strong and Weak Acids
There are two types of acids – strong acids and weak acids.

Acid Examples
Review this list of common acids.
| Name and Formula | Uses | General Information |
|---|---|---|
| Acetic Acid (CH3COOH) | Food preservation and commercial organic synthesis | Vinegar is about 5% acetic acid. |
| Acetylsalicylic (HOOC-C6H4-OOCCH3) | Pain relief, fever relief, reduce inflammation | It is the main component in aspirin. |
| Ascorbic acid (H2C6H6O6) | Antioxidant, vitamin | Vitamin C occurs naturally in some foods and is added to others. |
| Carbonic acid (H2CO3) | Found in carbonated drinks | It is found in acid rain and in cave formations. |
| Formic acid (HCOOH) | Leather processing, feed preservation, dissolves calcium carbonate | It is naturally found in bee venom and ant stings. |
| Hydrochloric acid (HCl) | Refine metal such as pickling steel to remove surface impurities | It is found naturally in gastric juice in the stomach. |
| Nitric acid (HNO3) | Fertilizers | It is colorless, but yellows when exposed to light. |
| Phosphoric acid (H3PO4) | Carbonated beverages, fertilizers, detergents | It has a slightly sour but pleasant taste. Detergents with phosphates cause water pollution |
| Sulfuric acid (H2SO4) | Car batteries, fertilizers manufacturing, drain cleaner, oil refining | It is highly corrosive. |

Bases
A base is a substance that produces hydroxide ions (OH-) when it is dissolved water. A base is any substance that accepts H+ hydronium ions from acids. They contain more negative ions than positive ions. In an aqueous solution, negative hydroxide ions are formed when a water molecule is split and one hydrogen atom is removed from the molecule.
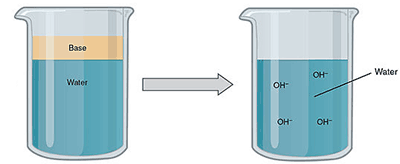
A base dissolves into an aqueous solution. Image attribution: Acids and Bases (Figure 3) by OpenStaxCollege and Connexions is licensed under CC BY 3.0. Cropped from original.
Bases are characterized by the following:
- Bitter taste
- Slimy or soapy feel on fingers
- Many react with acids and precipitate salts
- Turn red litmus paper blue
- Often contain metal oxides or hydroxides
Strong and Weak Bases
Strong bases are just as corrosive as strong acids. They can cause burns to skin.
We know that water can be written as H2O or H-O-H. When one hydrogen atom is removed, an OH- ion or hydroxide ion remains. Hydroxide ions can be just as reactive as hydronium ions, which make bases just as dangerous as acids.
In addition to strong bases, there are substances that are only slightly basic. There are a few foods that are slightly basic, such as:
- egg whites
- baking powder
Some medicines are bases, such as:
- milk of magnesia
- antacids
Most cleaners are bases, including:
- soaps
- drain cleaner
- laundry detergent
- oven cleaner
Soap is a mild base, which is used to help break the natural cohesion of water. This allows dirt and grime to be dissolved and washed away.


Base Examples
Review the following examples of common bases:
| Name and Formula | Uses | General Information |
|---|---|---|
| Aluminum hydroxide (Al(OH)3) | Color-fast fabrics, antacids, water purification | It is used in medicines that reduce phosphate levels in people with certain kidney conditions. |
| Calcium hydroxide (Ca(OH)2) | Leather making, mortar and plaster, lessen acidity of soil | It is called “slaked lime” because it's formed when calcium oxide (called lime) is mixed with water. |
| Magnesium hydroxide (Mg(OH)2) | Laxative, antacid | Milk of magnesia |
| Sodium hydroxide (NaOH) | Soap, oven cleaner, drain cleaner, textiles and paper | Known as lye and caustic soda, it generates heat when combined with water. It also absorbs moisture from the air and reacts with metals to form hydrogen (exothermic reaction). |
| Ammonia (NH3) | Cleaners, fertilizers, used to make rayon and nylon | It has an irritating odor that is damaging to the nasal passages and lungs. |

