Learn

Simple Chemical Reactions
There are thousands of known chemical reactions. Some are more simple and straightforward while others are more complicated. For the purpose of this course, we will focus on simple chemical reactions involving main group elements. Remember where the main group elements are located on the periodic table? They can be found in Groups 1-2 and Groups 13-18.
See larger version of the periodic table here.
Synthesis Reaction
In a synthesis reaction, two or more substances (the reactants) combine to form a single substance (a new product).

The combination of hydrogen (H) and oxygen (O) molecules to form water is a synthesis reaction. It's written: 2H2 + O2 → 2H2 O.
Other examples include:
- C(s) + O2(g) → CO2(g)
- H2O(l) + SO3(g) → H2SO4(aq)
- Remember, the letters in the parentheses tell you the form of the substance. s = solid, l = liquid, g = gas, and aq = aqueous.
Decomposition Reaction
In a decomposition reaction, one substance (a single reactant) breaks down into 2 or more substances (products). Basically the exact opposite of synthesis!
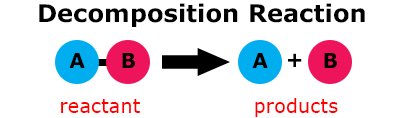
When nitrogen triiodide (NI3) is dry, it is very reactive. Watch the video Nitrogen Triiodide Detonation (0:23) to see the substance rapidly decompose into nitrogen gas (N2) and iodine gas (I2). The formula for this reaction is: 2NI3 → N2 + 3I2.
Other examples include:
- H2CO3(aq) → H2O(l) + CO2(g)
- CaCO3(s) → CaO(s) + CO2(g)
Single-Replacement Reaction
In a single-replacement reaction, a single element replaces a similar element of an adjacent reactant compound.
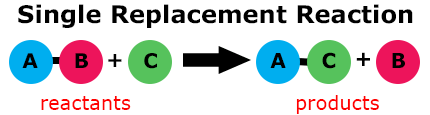
For example, magnesium (Mg) reacting with copper sulfate (CuSO4) will replace copper. This creates the compound magnesium sulfate (MgSO4) and copper (Cu), as shown in the formula: Mg + CuSO4 → MgSO4 + Cu.
Another example is:
- Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
Double Replacement Reaction
In a double replacement reaction, elements in two compounds switch places. Two ionic compounds exchange ions, producing 2 new ionic compounds.
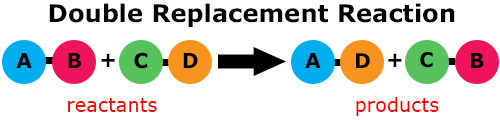
Zinc sulfide (ZnS), as a solid, will react with hydrochloric acid (HCl) to form zinc chloride (ZnCl2) and hydrogen sulfide (H2S), as gas. This double replacement reaction equation is written ZnS(s) + 2HCl(aq) → ZnCl2(aq) + H2S(g).
Other examples include:
- NaCl(aq) + AgNO3(aq) → NaNO3(aq) + AgCl(s)
- HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
Don't worry if you don't know each of these four types yet! You will continue to be exposed to these in future science course. The goal of this lesson is to give you an idea of what simple chemical reactions do and what they look like when written as equations.

Combustion Reactions
In addition to the simple chemical reactions you just learned about, there's one more specific type of chemical reaction you need to know: combustion reactions. Watch The Combustion of Wood (1:26) for an introduction.
As we learned, combustion is basically a form of burning a substance in the presence of oxygen. A combustion reaction occurs when a single element or compound reacts or combines with oxygen gas to produce energy in the form of heat and light. This rapid oxidation is called burning. Usually the substance being burned is some type of carbon (C) containing compound.
The products of a combustion reaction are always carbon dioxide (CO2) and water (H2O) so these types of reactions are easy to pick out in a group. A typical combustion reaction resembles the following:
Carbon containing compound + O2 → H2O + CO2
The carbon-containing compound can vary but the rest of the equation stays the same. Look at the following example:
CH4 carbon-containing compound + 2O2 oxygen → 2H2O water + CO2 carbon dioxide
Notice the reactants are a carbon-containing compound (CH4) and oxygen (O2) and the products are water (H2O) and carbon dioxide (CO2).
Other examples include:
- C(s) + O2(g) → CO2(g) + energy
- 2Mg(s) + O2(g) → 2MgO(s) + energy