Learn
![]()
Chemical Bonds
In a previous lesson, you learned that each element has its own unique chemical symbol. When atoms join together to make compounds, they are either sharing or transferring electrons to form a chemical bond. Often, when elements join to become a compound, the properties of that compound are not anything like those of the original elements.
For example, when potassium — a silver metal — bonds with iodine — a purple gas — at room temperature, it turns into a white crystalline salt. The formula for the newly formed compound is potassium iodide, or KI.
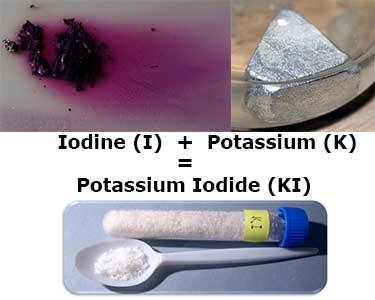
Iodine and potassium form the compound potassium iodide (KI), which possesses different properties than either original substance.
Compounds
Watch the video How Elements Form Compounds (3:22) to learn more. Login information.
Next, watch the video Compounds (1:52) to learn more about compounds. Login instructions.
![]()
Formulas
A chemical formula shows what elements are contained in a compound and the exact number of the atoms of each element in a unit of that compound. KI is the chemical formula for potassium iodide. When a compound contains more than one atom of a given element, a subscript is written after the element to show the number of atoms of that element in the compound. Look at CO2, which is carbon dioxide. CO2 has one carbon atom and two oxygen atoms. The subscript — in this case, the small 2 after the symbol for oxygen in the formula CO2 — tells us how many atoms of that element are in a single unit of that compound.
Watch the video “Chemistry in Focus: Chemical Formulas” (5:32) to learn more about writing chemical formulas. Login instructions.
| Common Name | Chemical Compound | Chemical Formula |
|---|---|---|
| milk of magnesia | magnesium hydroxide | Mg(OH)2 |
| table salt | sodium chloride | NaCl |
| cane sugar | sucrose | C12H22O11 |
| vinegar | acetic acid | CH3COOH |
| laughing gas | dinitrogen oxide | N2O |
| battery acid | sulfuric acid | H2SO4 |
| baking soda | sodium bicarbonate | NaHCO3 |
| sugar | glucose | C6H12O6 |
Atoms form compounds because they want to have a stable arrangement of electrons. Recall the electron dot diagram from a previous lesson. Those atoms with one or two valence electrons will be more reactive than atoms with six or seven valence electrons. All atoms want to have a full eight electrons in its outer shell like the noble gases have.

Here the first twenty elements on the periodic table are shown with their electron shells.
![]()
Bonding
A chemical bond is the force that holds atoms together in a compound. When atoms share, lose, or gain electrons, an attraction forms between the atoms, pulling them together to form a chemical bond. When atoms lose or gain an electron they are considered to be an ion. An ion is a charged particle that has either more electrons or fewer electrons than protons.
- Atoms that lose one or more electrons are positively charged and are known as cations.
- Atoms that gain one or more electrons are negatively charged and are known as anions.
In the figure below, the one valence electron from a sodium atom is shared with the seven electrons of a chlorine atom. When sodium loses the electron, it makes it a positively charged sodium ion. When chlorine gains an electron, it makes it a negatively charged chlorine ion. This process of bonding produces a stable compound of sodium chloride, also known as table salt. In the end, the overall sodium chloride compound is neutral since the charges from the two ions cancel each other.
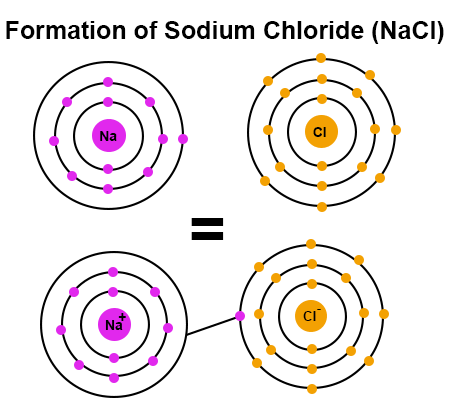
In the image above, the sodium atom has shared its one valence electron with the chlorine atom, making the sodium ion positively charged and the chlorine ion negatively charged. However, the whole NaCl compound formed by the bond is neutral and has no overall charge.
![]()
Types of Bonding
There are three types of bonds that can form between atoms.
- Metallic bonds are formed when metal atoms share all of their electrons in a kind of electron cloud within the metal. This type of bonding allows metal wires to be good conductors of electricity.
- Covalent bonds are formed when non-metal atoms, which hold their electrons strongly, come together and share the valence electrons between them. Carbon has four valence electrons and can share these electrons with up to four different atoms. Covalent bonds are flexible, allowing covalent compounds to bend and stretch without breaking. Our bodies and all living things are made mostly of covalent compounds of carbon.
- Ionic bonds are formed from positive and negative ions, which are atoms that have gained or lost electrons to become electrically charged. Opposite electrical charges attract, so positive ions join with negative ions to form electrically neutral compounds.
Open in New Window (Larger Version)
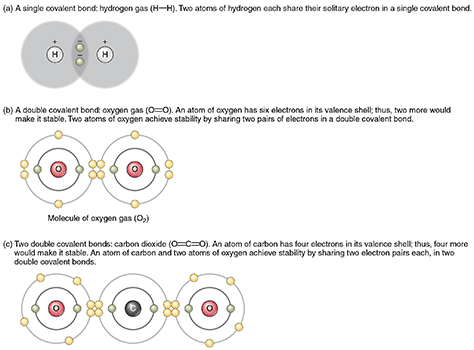
Covalent bonds between hydrogen atoms, oxygen atoms, and carbon dioxide. Image attribution: Covalent Bonding by OpenStax College and Connexions is licensed under CC BY 3.0.
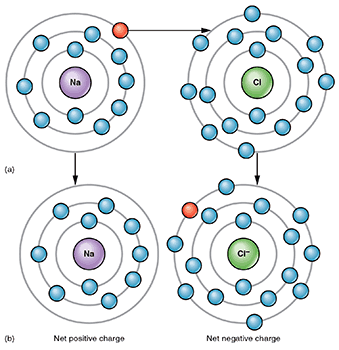
Ionic bonding occurs when an atom loses an electron to another atom, making each atom an anion (negative charge) or cation (positive charge). Image attribution: Ionic Bonding by OpenStax College and Connexions is licensed under CC BY 3.0. Image has been cropped.
Unequal sharing of electrons
A polar bond occurs when electrons are shared unequally resulting in a slightly positive end and a slightly negative end. When atoms bond with each other, some atoms will hold the electrons more closely than the others. Those atoms that hold the electrons more closely will always have a slightly negative charge. A polar molecule occurs when unequal sharing of electrons results in a slightly positive end and a slightly negative end, keeping the overall molecule neutral. A nonpolar molecule does not have oppositely charged ends.
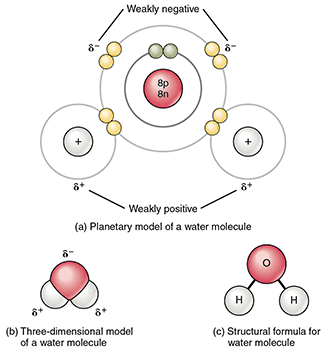
A water molecule has a polar covalent bond. Image attribution: Polar Covalent Bonds in a Water Molecule by OpenStax College and Connexion is licensed under CC By 3.0.
Watch the video Types of Bonding (21:29) for a complete review of chemical bonding. Login instructions.

