
Scientists learned early on that gases dissolve into liquids faster at lower temperatures and higher pressures. When the pressure is released, the gas is no longer held in solution and bubbles begin to form. Mentos candy has a rough sugar coating that attracts the gas bubbles and causes them to come out of solution at a very rapid rate. The gas has nowhere to go but up and shoots out the top of the bottle carrying the liquid with it which forms a very cool geyser
What other things affect the rate of solution?
Stirring: When you stirred the sugar water in lesson 7.01, what happened? (more sugar dissolved) Stirring has two effects. First, it moves the particles of water and solute around and causes more of them to come into contact and increases the rate of dissociation. Secondly, it adds energy to the particles. We know that as energy increases, particle motion increases. Increasing the speed of the particles increases the mixing of the particles and allows more to dissolve.
Size of the particles: What dissolves faster, a sugar cube or sugar granules? The sugar granules, of course. Why? The sugar granules are separated into more particles which can interact more rapidly with the water molecules than a solid block of sugar which has all of the sugar molecules held together in the cube. If you want to dissolve a solid into a liquid faster, grind the solid into powder. Try this! Compare the rate of dissolving of powdered sugar with the rate of dissolving of granulated sugar.
Temperature: Did the ice water dissolve the sugar faster than the warm water? No. Why? The molecules of water in ice water are moving much slower than the molecules of warm water so the dissociation of the particles of solute occurs at a much slower rate. If you want to make really sweet tea, add the sugar to the tea before the ice.
Pressure: The makers of soda pop learned early on that gas under pressure dissolves more rapidly. Why? The gas particles under pressure are forced together and when they are placed into the liquid, the pressure of the liquid is less, allowing the gas particles to push apart and spread throughout the liquid faster than they do at normal pressure.
Solubility Curves: Scientists have developed graphs of the rate of dissolving of many different substances and use these graphs to determine the amounts of solutes required to make saturated solutions. The graph below compares the solubility of different substances in water. Notice that the graph compares the temperature of the water to the mass of the solute. The mass of the water is 100 grams. The density of water is about 1 g/ml so 100 grams of water would have a volume of 100 ml.
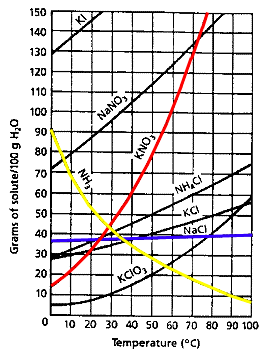
NaCl (table salt): find the line for NaCl on the graph. Notice that it doesn’t change very much. About 37 grams of salt will dissolve in 100 ml of water at 0 degrees C and 41 grams of salt will dissolve in water at 100 degrees C.
KNO3 (potassium nitrate): Find the line for KNO3. Notice that about 13 grams will dissolve at 0 degrees C and 150 grams will dissolve at 75 degrees C. Above 75 degrees C, the potassium nitrate will melt and actually become the solvent for the water!
NH3 (ammonia): Find the line for NH3. 90 grams dissolves at 0 degrees C but only 8 grams dissolves at 100 degrees C! Ammonia actually becomes more dense at higher temperatures and does not dissolve easily in hot water.
From the chart we can conclude that most substances dissolve more easily when the temperature is increased.
