Learn

Electric Current

Electronics, such as an alarm clock, lights, and stereos, work when there is an electric current flowing through them. An electric current is the rate that the electric charges move through a wire in a single direction.
- The symbol I is used for current.
- Michael Faraday designated this symbol based on the Greek word ion which means going.
- The SI unit of current is the ampére (A). One ampére equals to one coulomb of electric charge moving past a point in one second (C/s).

Electric current differents by state or type of matter.
- In metals, the current is made of moving electrons.
- In gases and many chemical solutions, the current is carried by moving ions, which can be positively or negatively charged.
Watch GPB Segment E: Current Electricity (8:45).

Direction of Flow
Let's look at the flow of electrons and current in flashlights and other battery-operated devices:
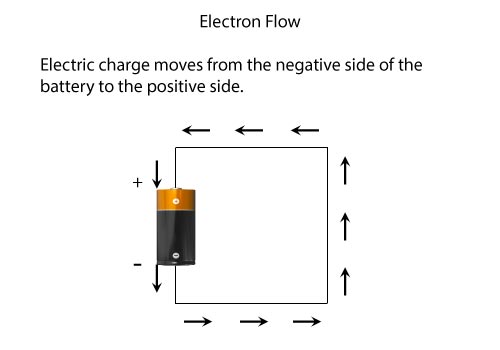
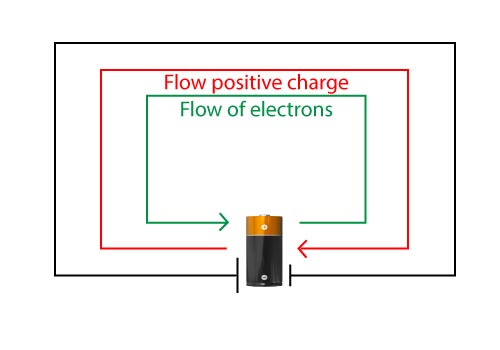
- The electrons flow from the negative battery terminal to the positive terminal.
- The current flows from positive to negative. Current is the direction that positive charges flow.

Voltage
Electric current flows from higher voltage to lower voltage. The voltage difference, also called potential difference, is the change in the electrical potential energy of a charged particle divided by its charge.
- This occurs when a charge moves from one place to another in an electric field.
- The symbol for potential difference is the V and is often called voltage.
- The SI unit for voltage is V (volt).
- The volt is equal to one joule per coulomb (1 J/C).

Electric Cells and Batteries
Batteries are typically the most common example given of electric cells, which are devices used to generate electricity. Did you know that batteries actually consist of two or more cells, which are connected in a specific pattern?
- An electric cell usually consists of two electrodes. Electrodes are made from material that can conduct electricity.
- Along with the electrodes, there is also an electrolyte, a liquid or solid that separates the two electrodes and contains ions.
- These parts allow the flow of electricity inside the cell.
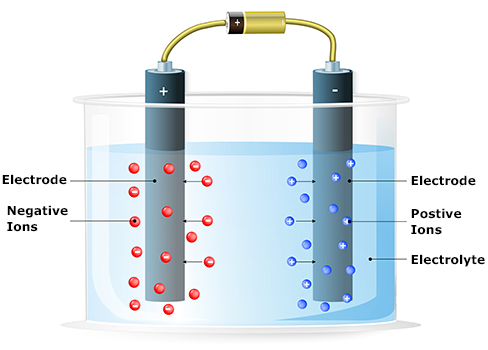
Optional: Take a look at the Simple Electric Cell simulation. Scroll down to the electric cell animation. Use the slider to speed up and slow down the activity of the cell. Looking over this simulation, you can tell that there is a lot more going in within an electric cell than we covered! You will learn more about the complexity of electric cells in later classes, like chemistry. For this course, be sure to focus on how the animated diagram shows the activity of the electrodes in the electrolyte.
Types of Electric Cells
There are at least two types of electric cells you should be familiar with:
- Wet cell - an electric cell in which the electrolyte is a liquid (hint: the Simple Electric Cell simulation and the electrolysis diagram above both show wet cells
- Dry cell - an electric cell in which the electrolyte is more similar to a paste than a liquid. Because it uses a paste, there is really only enough moisture in the electrolyte to allow an electric current to flow through.
We're going to focus more on dry cells in this unit, as they are the most commonly used electric cells today. You can find them in alkaline batteries.
More About Batteries
Batteries convert a chemical reaction into electrical energy. Dry cell or alkaline batteries - like the AA, AAA, C, D, and 9 V batteries that are common household batteries - can differ in several ways, but they all have the same basic components, as seen in the dry cell diagram below. Read How Do Batteries Work from Energizer to learn about these components.
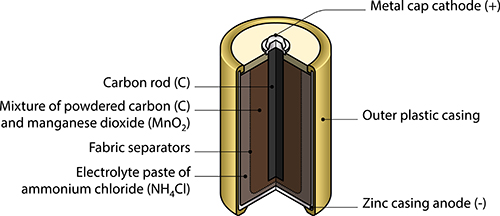
In this dry cell, the electrolyte is a paste made of ammonium chloride.
These batteries can also be made from different materials, such as:
- Carbon
- Zinc
- Mercury
- Lithium
- Zinc


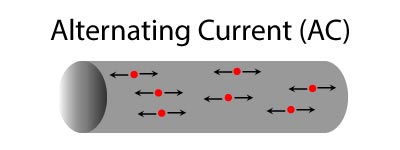
Alternating Current and Direct Current
There are two types of current:
- Alternating Current (A/C)
- Direct Current (D/C)
Alternating current changes direction 120 times each second.
- In the U.S., all household appliances, such as, hair dryers, coffee pots, and toasters are all built to use alternating current.
- Nikola Tesla invented the alternating current in 1888.
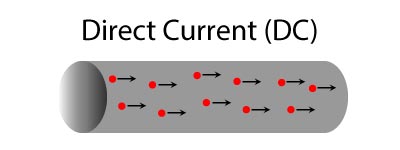
- Electronics such as cell phones, portable stereos, DVD players all use direct current.
- Dry cell batteries also use direct current.
When charging your cell phone or other electronics, you use an AC/DC adapter. An AC/DC adapter converts the AC current from the outlet to DC current that your electronic device can utilize.

An AC/DC adapter like the one above is used to power and/or charge an electronic device.

