Learn
Law of Conservation of Mass
The Law of Conservation of Mass states that matter cannot be created or destroyed. It can just change forms.
Watch this video on the Law of Conservation of Mass (6:57) to get started. Find video Login information here.
Our goal is to apply the Law of Conservation of Mass to chemical reactions.
In a chemical reaction, atoms can rearrange to form new compounds, but atoms cannot be created or destroyed. This means that if we have 4 atoms of hydrogren in the reactants, there must be 4 atoms of hydrogen in the products. Look at the equation below and see if you can identify the 4 hydrogen atoms in the reactants and the 4 hydrogen atoms in the products. Those hydrogen atoms may be bonded to new elements but they cannot disappear.
CH4 + 2O2 → 2H2O + CO2
This is why chemical equations are balanced – it ensures that the law of conservation of matter is followed and that the equation shows that no atoms were created or destroyed during the process. Look at the equation below and we will see how there is the same number of atoms on each side.
CH4 + 2O2 → 2H2O + CO2
- 1 C atom in the reactants and 1 C atom in the products
- 4 H atoms in reactants and 4 H atoms in the products (2x2=4)
- 4 O atoms in the reactants and 4 O atoms in the products
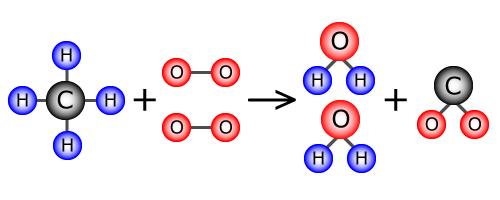
You do not need to be able to balance equations yet, but you do need to be able to recognize the purpose of a balanced equation.
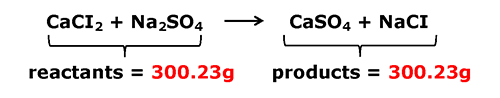
Besides balanced equations, Law of Conservation of Mass also means that the products of a chemical reaction should always have the same mass as the reactants. If the reaction begins with 150.0g of reactants, the products should add up to 150g at the end of the reaction.
Look at the example below. The reactants added together are 300.23g, so therefore the total mass of the products must also be 300.23g. This way mass is conserved.
Now look at this example:
Ca + ZnCO3 → CaCO3 + Zn
64g 192g 152g ?
Based on the Law of Conservation of Mass, how much Zn should be produced? Well, we know that the mass of the products must be the same as the reactants.
- The reactants added together are 256g (64g Ca + 192g ZnCO3).
- Therefore, the products must also add up to 256g.
- Right now, the first product is 152g, meaning that Zn must be 104g (256g total - 152g CaCO3 = 104g Zn).
As a side note, often times in lab, some products are lost during the reaction. Some liquid may splash out of a beaker or some bubbles may cause gas to be released to the air. If things like this happen, the mass of the products may appear to be less than the reactants but in reality some of the mass was simply lost to different areas. In a perfect lab, the mass of the products will be the same as the mass of the reactants.