Learn
Reactions
A chemical reaction is a change in which one or more substances are converted into new substances. In a chemical reaction, whatever substance you begin with is chemically changed into a different substance at the end. You will need to be able to identify when chemical reactions have occurred by examining the properties of the substances before and after a change. Signs of chemical reactions include the formation of a new substance (For example, a gas is produced from two liquids) or the disappearing of a substance. Chemical reactions often involve the formation of chemical bonds, the breaking of chemical bonds, or a combination of both breaking and forming chemical bonds to produce new substances.
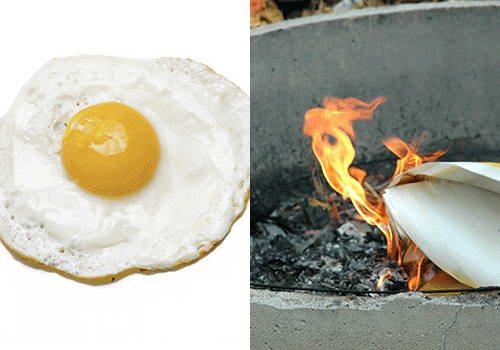
Think about the examples that were given in the introduction: frying an egg or burning wood or paper. For each of these examples, what you have at the end of the chemical reaction is not the same substance that you had at the beginning. The wood or paper has become black and looks and feels much different than before. The egg, while it may still look similar, has become more white and thick.
Open Compounds: Chemistry Basics in a new window
Note: The presentation may take a moment to load.
Read Indicators of a Chemical Change.

So, would ripping a piece of paper apart be a chemical reaction?
Reactants and Products
A chemical reaction has two primary parts: the reactants and products.
Reactants are the substances present at the beginning of a chemical reaction, or the original substances.
Products are the new substances (from the reactants) in the chemical reaction.
The relationship between reactants and products can be written:
reactants → products
This way of writing a chemical reaction is often called a chemical equation. Notice the reactants are always written on the left, then an arrow followed by the products written on the right.
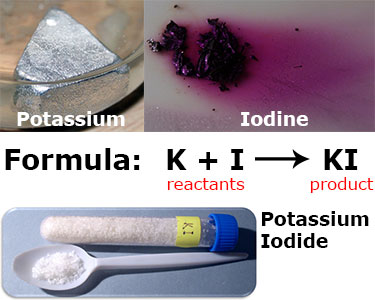
In this reaction, potassium (K) and iodine (I) are the reactants, and potassium iodide (KI) is the product. A simplified formula for this reaction is shown above, in reality, this reaction is written:
2K + I2 → 2KI
Notice here the reactants (K and I) are written on the left and the product (KI) is written on the right. That is the easiest way to identify which are the reactants and which are the products in a chemical equation.