Learn
Ionic Bonds
Now that you have a basic understanding of why atoms form bonds, let's discuss the first type of chemical bonding: ionic bonding. Ionic bonding occurs when electrons are transferred from one atom to another atom.
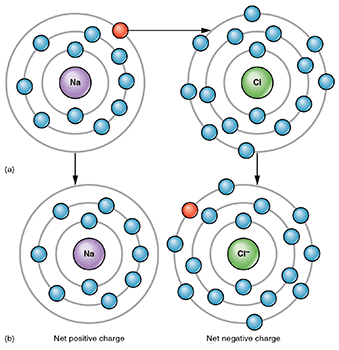
See larger image of sodium and chlorine atoms combining in an ionic bond to form sodium chloride here. Image attribution: Ionic Bonding by OpenStax College and Connexions is licensed under CC BY 3.0.
As you saw in the video, ionic bonding involves ions, which are charged atoms. Ions form because one atom gains electrons (and becomes negatively charged) and another atom loses electrons (and becomes positively charged). When one atom is negatively charged and the other is positively charged, they attract each other and form an ionic bond.
Metals tend to form positive (+ charged) ions because they have low numbers of valence electrons, which they can lose easily. Nonmetals tend to form negative (- charged) ions because they have a higher number of valence electrons so they tend to gain electrons easily. This means that ionic bonds usually form between a metal and a nonmetal.
For example, a sodium (Na) atom (a metal) and a chlorine (Cl) atom (a nonmetal) form an ionic bond where the sodium atom loses an electron to the chlorine atom. This leaves the sodium atom with a positive charge and the chlorine atom with a negative charge. The overall compound (sodium chloride) is neutral and no charge. (see image of sodiom and chlorine atoms combining in an ionic bond above)
Complete the Ionic Bonding interactive tutorial to review what you have learned so far. Enter your first name in the Guest Login box and select "Login" to begin.