Learn
Covalent Bonds
So far, you have learned about one type of bonding so far where atoms transfer electrons to become ions and those oppositely charged ions are then attract to each other. Now, let's examine a different type of bonding where, instead of transferring electrons, atoms share electrons to reach a full outer energy level. This is called covalent bonding.
As you saw in the video, atoms in a covalent bond share their valence electrons in order to have a full outer energy level. Atoms in a covalent bond are called molecules.
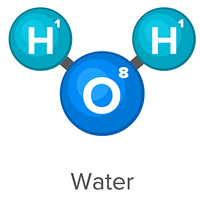
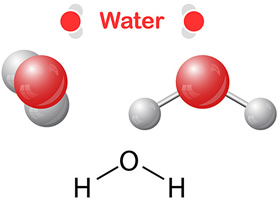
The water molecule (H2O) shown below contains two hydrogen atoms and one oxygen atom. They are sharing four electrons in a covalent bond.
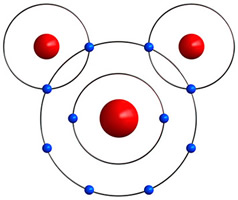
Covalent bonds form between 2 (or more) nonmetals. Since nonmetals have higher numbers of valence electrons, they tend to hold onto their electrons (instead of losing them), which makes them ideal for covalent bonding. Remember, nonmetals include Groups 14 - 16 and hydrogen in Group 1.
Complete the Covalent Bonding interactive tutorial to explore some different properties of covalent bonds and to review what you have learned. Enter your first name in the Guest Login box and select "Login" to begin.
Polar Covalent and Nonpolar Covalent Bonds
There are two types of covalent bond: nonpolar covalent bonds (which we just discussed in the last tab) and polar covalent bonds. You need to be able to tell the difference between these types.
Start by watching Chemistry: Challenges and Solutions - Polarity.
Polar Covalent
As you learned in the videos, polar covalent bonds occur when atoms do not share the electrons equally. The electrons spend more time around one atom which creates two oppositely charged poles — hence the name “polar.”

Water molecules (H2O) have polar covalent bonds.
Nonpolar Covalent
A nonpolar covalent bond occurs when atoms share electrons equally, and the electrons do not spend more time around either of the atoms.
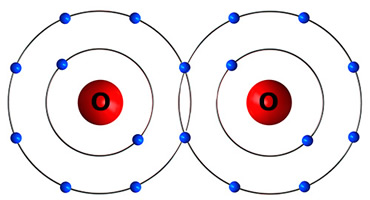
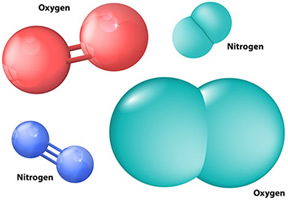
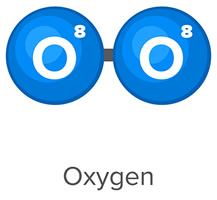
An oxygen gas (O2) molecule has a nonpolar covalent bond.
How do you know if a covalent bond is polar or not?
In general, if the two atoms sharing electrons are different elements, the bond will be polar. This is because the size and attraction of the atoms differ.
If the two atoms sharing electrons are the exact same type of atom, then the bond is nonpolar.
Your Turn
Let's look at a few examples. Determine if each molecule below has a polar covalent bond or a nonpolar covalent bond.
- Is an O2 molecule polar covalent or nonpolar covalent?
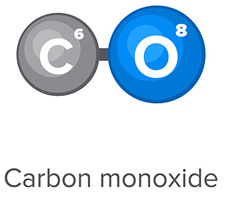
- Is a CO molecule polar covalent or nonpolar covalent?
- Is a H2O molecule polar covalent or nonpolar covalent?