Learn
Chemical Bonds
A chemical bond is simply a force that holds atoms in a compound together. There are two main types of chemical bonds we will learn about this unit: ionic bonding and covalent bonding.
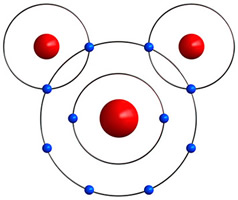
This water molecule is a combination of two hydrogen atoms and one oxygen atom all bonded together.
Often, when elements join to become a compound, the properties of that compound are not anything like those of the original elements. For example, when potassium (a silver metal) bonds with iodine (a purple gas at room temperature), it turns into a white crystalline salt. The formula for the newly formed compound is potassium iodide, or KI.

Iodine and potassium form the compound potassium iodide (KI), which possesses different properties than either original substance.
Why Elements Form Bonds
Before we start with ionic bonding, we need to look briefly at why atoms and element even want to form bonds in the first place. Complete the interactive:
As you saw in the videos, the goal of atoms is to get a full outer energy level — which is normally indicated by 8 valence electrons. This is sometimes called the octet rule
- Elements form chemical bonds in order to get 8 electrons in their outer energy level.
- They do this by either transferring or sharing electrons.

In a water molecule (H2O), oxygen has a full outer energy level of 8 electrons because it has bonded with hydrogen atoms.
If elements want to get 8 valence electrons by forming chemical bonds, which group on the periodic table is the LEAST likely to form bonds?