Learn
Valence Electrons
Periodic trends, or patterns in the periodic table, are prevalent all throughout the periodic table. It is important to note here that these patterns and trends we will be discussing are most clearly seen in the main group elements (elements in Groups 1-2 and 13-18).
Groups 3-12 do not follow the periodic patterns and trends as easily. This has to do with the way their electrons are arranged in the atom, but you will learn more about that in a chemistry course one day!For now, just realize when we talk about a pattern or trend, we are talking about the main group elements.
The first pattern we will discuss are valence electrons. Valence electrons are the outer most electrons in an atom. They are the ones that are furthest from the nucleus.
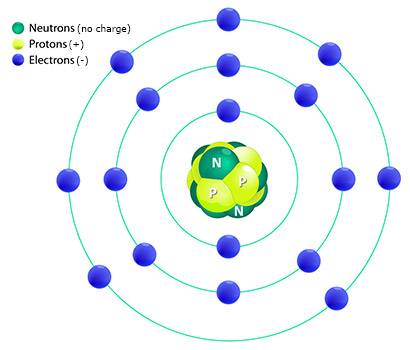
The electrons in the outer ring of this atom are the valence electrons.
In our example image above, the electrons are shown in different energy levels. The electrons that are in the level furthest from the nucleus are considered valence electrons. So, this atom has 7 valence electrons.
Valence electrons are important because, since they are the outermost electrons, they are the electrons that interact with other atoms. Therefore, they are responsible for most of the properties (characteristics or behaviors) of an atom. In general, an atom can have from 1-8 valence electrons.
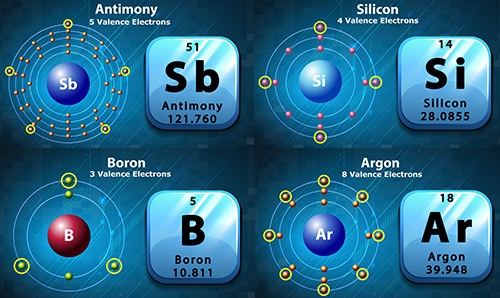
Look at the different elements shown above. They each have different numbers of valence electrons (circled in yellow on the image). See a larger version of the image here.
Pattern of Valence Electrons
Let's examine the pattern of valence electrons in the Periodic Table.

See a larger version of the periodic table here.
The number of valence electrons for each element actually varies directly with the group the element is in. The group number helps indicate the number of valence electrons. Review the list below to see this pattern:
- Group 1 has 1 valence electron.
- Group 2 has 2 valence electrons.
- Group 13 has 3 valence electrons.
- Group 14 has 4 valence electrons.
- Group 15 has 5 valence electrons.
- Group 16 has 6 valence electrons.
- Group 17 has 7 valence electrons.
- Group 18 has 8 valence electrons.
Notice that we skipped Groups 3-12. Again, it's because these groups do not follow the predicted patterns well.
The periodic trend of the number of valence electrons being linked to an element's group number only works for Groups 1-2 and 13-18.
How to Determine
To find the number of valence electrons, we simply determine what group an element is in. For example, the element sodium (Na) has 1 valence electron because it is in Group 1.
Now you try. How many valence electrons does sulfur (S) have?
Answer: Sulfur has 6 valence electrons because it is in Group 16. Remember, don't confuse valence electrons with total electrons! Sulfur has 14 total electrons (based on its number of protons) and then 6 of these are valence electrons.