Learn
The Periodic Table
The Periodic Table of Elements is a way to organize known chemical elements. Remember, elements are pure substances that are composed of the same type of atom. The periodic table is full of information.
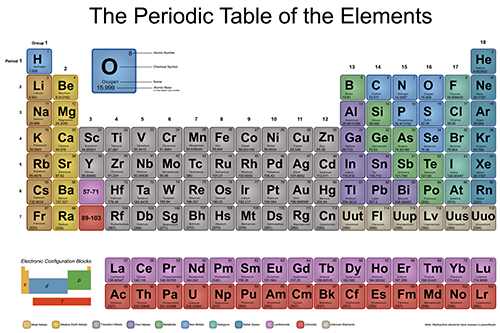
Your goal in this unit to know how to use the information on the periodic table to correctly identify different properties characteristics of elements.
As we begin, take a look at the video Developing The Periodic Table (3:27) to learn about the history and development of the Periodic Table.
Next, to learn about the basic organization of the periodic table, check out Patterns and Trends in the Periodic Table. Pay close attention (you may even want to take some notes!) and come back to answer the questions below.
Review
Answer the following questions to check your understanding of what you've learned so far in the videos in this lesson.
- What are the horizontal arrows on the periodic table called?
- What are the vertical columns on the periodic table called?
- What does the atomic number tell you?
- How many periods are there in the periodic table?
- How many families or groups are on the periodic table?
- What element begins (is at the top of) Group 17?
- Find Period 3. What element begins this period?
To answer the next questions, you will need to open the larger version of the periodic table.
Elements on the Periodic Table
There are three main types of elements which compose the Periodic Table:
- Metals
- Nonmetals
- Metalloids
Each type has different properties and characteristics.
Metals
Metals tend to be:
- Malleable: able to be bent into shapes without breaking
- Ductile: able to be drawn into a wire
- Conductors: allow heat and electricity to pass through
- Solids
- Shiny


Metal jewelry and copper wire are made of different metals.
Nonmetals
Nonmetals tend to be:
- Brittle: easily broken
- Not ductile: cannot be stretched into wires or sheets
- Insulators: restrict the flow of heat and electricity
- Not shiny
- Not solid


Oxygen gas and sulfur powder are nonmetals.
Metalloids
Metalloids are harder to categorize as they have properties of both metals and nonmetals.
For example, some metalloids will conduct electricity under certain conditions, while others will not. It really just depends on the metalloid.

Examples of different metalloids
Location on the Periodic Table
Metals, nonmetals, and metalloids each have specific locations on the periodic table. Let's look at what groups contain each type of element.
- Metals: Groups 1 - 12
- Metals are located mainly on the left hand side of the Periodic Table in Groups 1-12.
- However, notice that hydrogen is on this left side but is a nonmetal.
- Metals: Part of Groups 13 - 15
- Some metals are also located near the bottom of Groups 13-15. These include metals like aluminum and tin.
- Nonmetals: Groups 14 - 18
- Nonmetals are located mainly on the upper right side of the Periodic Table in Groups 14-18.
- Of course, don't forget hydrogen in Group 1!
- Metalloids: Staircase
- Metalloids form a stair-step type line between the metals and nonmetals.
- These include elements like silicon and germanium.
- Main Group Elements
- The elements in Groups 1-2 and 13-18 are considered main group elements.
- These elements behave in the most predictable ways.