Learn
The Composition of Atoms
Elements
We've been looking at the Periodic Table as a whole; now let's zoom in to look at the individual boxes, which represent elements.
We will review the parts of an atom and then discuss how information on the Periodic Table is related to these subatomic particles.

Atoms
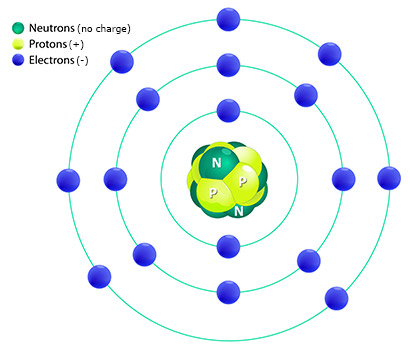
Remember from your past science courses that atoms are composed of three primary particles:- protons
- neutrons
- electrons
These are referred to as subatomic particles.
Subatomic Particle Charges
Protons are positively charged and located in the nucleus.
Neutrons are neutral have no charge and located in the nucleus.
Electrons are negatively charged and located orbiting around the nucleus.
Elements
What do subatomic particles and the Periodic Table have to do with each other?
Take a look at this example block from the Periodic Table. It's the element chlorine. There's a lot of information about the element chlorine in this square!
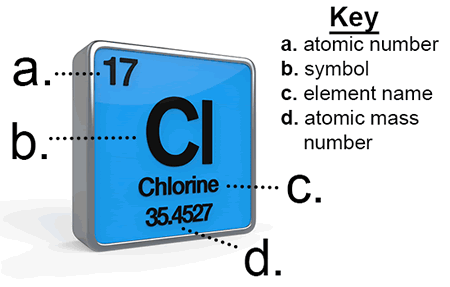
For each element on the Periodic Table, the following information is given:
- the atomic number
- the atomic symbol
- the element name
- atomic mass number
We're going to discuss all 4 parts in detail.
Atomic Number
The whole number listed for an element is the atomic number. It tells us the number of protons present in an atom of that element.
In our example, the atomic number is labeled with the letter "a."
In this case, chlorine has an atomic number of 17, meaning there an atom of chlorine has 17 protons.
Atoms are usually neutral, which means they don't have a charge. Therefore, if an atom of chlorine has 17 protons (which are positively charged), it must also have 17 electrons (which are negatively charged). This way the positive and negative charges balance to give a neutral atom.
So, the atomic number also tells you the number of electrons, as long as the atom is neutral.
Let's test your understanding of atomic numbers. Based on the periodic table entry for the element oxygen listed below, answer the following questions:

- How many protons does an atom of oxygen have?
- How many electrons does a neutral atom of oxygen have?
Atomic Symbol and Element Name
Next, the periodic table entry for an element will list the atomic symbol. On our example, it is labeled with "b." The symbol Cl stands for chlorine and is used as a shorthand notation for the element.
Most periodic tables will also list the element's name. On our example, it is labeled "c."

Atomic Mass
The last bit of information normally included on the periodic table for each element is the atomic mass. It is the average of all the different masses of that element that exist in nature, which is why this number appears as a decimal.
In our example, the atomic mass is labeled with the letter "d."

We know the atomic mass is a decimal because it is an average.
In reality, the mass of a single atom is usually: the number of protons + the number of neutrons.
Each proton and neutron weighs about 1 amu amu = atomic mass unit , while the electron is so small it does not contribute to the mass.
Rounding the Atomic Mass
The number of protons plus the number of neutrons is often reported as the mass number of an element, which is commonly written as a whole number after an element name. An example of this is "chlorine-35."
The most common way to get a mass number is to round the atomic mass listed on the periodic table. Since chlorine's atomic mass is 35.45, we would round down to 35.
Remember rules for rounding:
- Any number .4 and below, round down to the nearest whole number.
- Any number 0.5 and above, round up.
Example: Atomic Mass Rounding
Chlorine-35 represents an atom of chlorine with a mass number of 35. We can figure out the following information:
- Chlorine-35 has 17 protons because its atomic number is 17
- Chlorine-35 has 17 electrons because, as a neutral atom, the number of protons = number of electrons
- Chlorine-35 has a mass number of 35. Since mass number = protons + neutrons, we can determine that it has 18 neutrons (17 protons + 18 neutrons = mass number of 35)
Your Turn
Now, it's your turn! Use the entry for the element nitrogen to determine the following:

- How many protons does nitrogen-14 have?
- How many electrons does nitrogen-14 have?
- How many neutrons does nitrogen-14 have?
- What element has 12 protons?
- What element has a mass number of 19 and 10 neutrons?
You can use these numbers to determine the element using a periodic table. Open a larger version of the Periodic Table here.