Learn
States of Matter
We know that matter is everything that has mass and takes up space. Matter is made up of atoms and molecules and can be categorized into different types. Matter also has different states.

Watch the video States of Matter (3:03) below to learn more.
States of Matter: Video Short | Science Trek downloaded from PBS LearningMedia, https://www.pbslearningmedia.org/resource/idptv11.sci.phys.matter.d4kmat/states-of-matter/. Rights to use this asset do not expire. Asset Copyright: ©2005 Idaho Public Television. All rights reserved. Source: Science Trek: "States of Matter". Project funded by: Laura Moore Cunningham Foundation. See full license here.
4 States of Matter
The four states of matter are:
- Plasma
- Solid
- Gas
- Liquid

Solids
Solids are hard and maintain their own shape.

Liquids
Liquids are fluid and take on the shape of their container.
Gases
Gases move freely and take the shape of their container.
Plasma
Plasmas are similar to gases, but their atoms are different. On Earth, we find plasma in lightning. Plasma makes up most of the matter in the universe, including the stars.
Physical States
The physical state of the atoms and molecules determine the state of the matter. There are distinct differences in the states of matter.
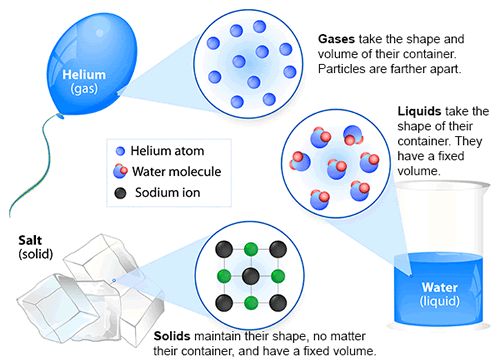
Examples of three of the four physical states.
Phase Changes
Because matter is based on the physical state of its atoms and molecules, they can change from one form to another. This is called a change of state or phase change.
- A solid can be heated to become a liquid.
- A liquid can be heated to become a gas.
We can see the change from solid to liquid, but we cannot always see the liquid change to a gas.
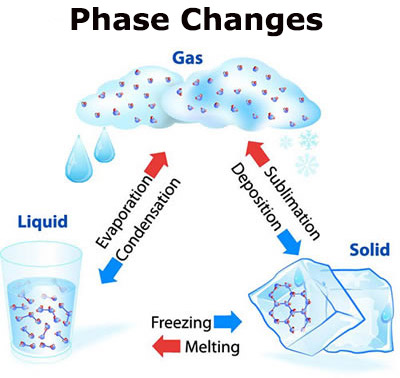
Matter can change state through processes like evaporation, condensation, and the others listed.
Chemical State is Stable
Even though matter changes physical states, its chemical state remains stable and does not change.
A good example of matter changing physical states but keeping its chemical state is water.
- Water can be a liquid and freeze to form a solid (ice).
- Ice can melt back to liquid water.
- Both can evaporate to become a gas.
During these changes in physical state, the chemical state does not change. It is always water (H2O).
Temperature and Plasma
Temperature affects the physical state of matter. As temperature increases, a gas can ionize to become plasma. Conversely, as temperature decreases, plasma can undergo recombination (also called deionization) to become gas.
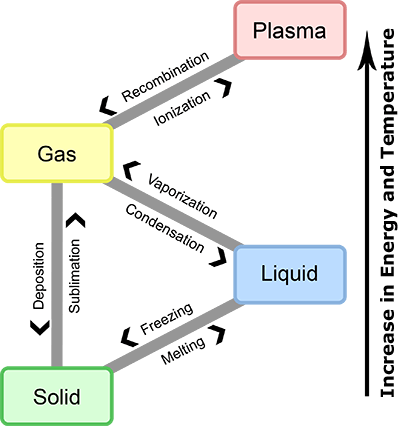
Particulate Nature of Matter
To learn more, complete The Particulate Nature of Matter activity.
Complete both parts:
- The Tutorial
- The Problem Set
Pay close attention to how the particles in solids, liquids, and gases move, as you will need this information to complete the Task.