Learn
Different Types of Matter
Matter is everything you see, everything you can't see, and everything that has mass and takes up space. All matter is made of atoms and molecules. We can group matter into different categories based on certain properties and characteristics.
Below is a graphic organizer that shows the relationships between different types of matter.
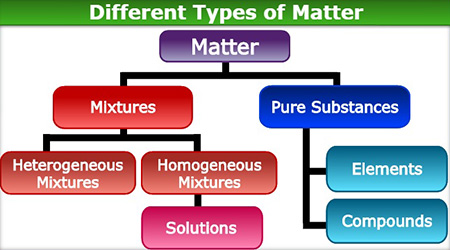
The different types of matter on this graphic organizer are explained below.
Matter
- Definition: Matter is anything that has mass and takes up space.
- Matter is everything you see, everything you cannot see, and everything that has mass and takes up space. All matter is made of atoms and molecules.
- We categorize matter into mixtures and pure substances.

Matter is everything you can see and cannot see!
Pure Substances
- Definition: A pure substance is matter that is composed of one kind of particle throughout the substance. It has a fixed composition. Therefore, it always has the same properties.
- Pure substances can be either:
- elements
- compounds

Copper is an element; therefore, it is a pure substance.
Elements
- Definition: Elements are pure substances that contain all of the same type of particle, called atoms. There are few elements found alone in nature.
- See examples of elements in everyday items below:
- Neon (Ne) is used in neon signs.

- Titanium (Ti) is used in golf clubs.

- Magnesium (Mg) is used in aerospace manufacturing.

- Copper (Cu) is used in electrical wiring.

- Aluminum (Al) is used in automobile manufacturing.

- Neon (Ne) is used in neon signs.
Compounds
- Definition: A compound is a pure substance containing particles called molecules. Molecules are made of atoms of two or more elements. Most elements combine with other elements to form compounds.
- See examples of everyday compounds below:
- Carbon dioxide (CO2) is colorless gas.

- Limestone is calcium carbonate (CaCO3).

- Salt is sodium chloride (NaCl).
- Ammonia (NH3) is used in cleaning.

- Water is made of hydrogen and oxygen atoms combined together to form molecules. It's chemical formula is H2O (said "H two O").

- Carbon dioxide (CO2) is colorless gas.
Mixtures
- Definition: A mixture is a substance that contains two or more pure substances. Therefore, it has two or more kinds of particles.
- There are two categories of mixtures:
- heterogeneous mixtures
- homogeneous mixtures

Trail mix is a kind of mixture.
Heterogeneous Mixtures
- Definition: A heterogeneous mixture has distinct particles of two or more substances that do not mix completely together.
- Some everyday examples of heterogeneous mixtures are salsa, pizza, and chili.

Fruit salad is a heterogeneous mixture.
Homogeneous Mixtures
- Definition: A homogeneous mixture has particles so small that they cannot be seen without a microscope.
- This type of mixture remains constantly and uniformly mixed together.

Iced tea is a homogeneous mixture.
Solutions
- Definition: Solutions are mixtures of two or more substances that appear to be made of one substance
- All solutions are homogeneous mixtures.

Because sweet tea is a homogeneous mixture, it is also a solution.