Learn
Chemical Reactions
Chemical reactions take place all around us. A chemical reaction is a change in which one or more substances are converted into new substances. When substances mix together, there are:
- Reactants: The substances present at the beginning of a chemical reaction, or the original substances.
- Products: The new substances (from the reactants) in the chemical reaction.
The relationship between the reactants and products can be written:
reactants → products
Watch the following videos to learn more. Login instructions.
- What are Chemical Reactions? (1:30)
- Traits of Chemical Reactions (2:34)
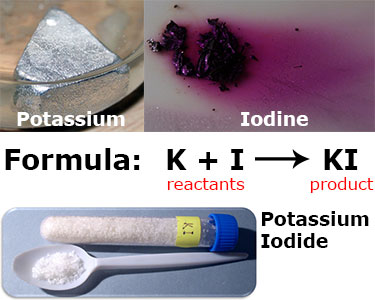
Potassium (a silver metal) reacts with iodine (a purple gas) to create KI, or potassium iodide, a white crystalline salt.
In this reaction, potassium (K) and iodine (I) are the reactants and potassium iodide (KI) is the product. A simplified formula for this reaction is shown above. In reality, this reaction is written:
2K(s) + I2(s) → 2KI(s)
For this lesson, you only need to know how to identify the products and reactants listed in a chemical formula. Later in this unit, you will learn what the numbers and parentheses in a chemical equation represent, but you don't need to worry about that just yet.
Energy in Chemical Reactions
Most chemical reactions are slow to show the reaction, but all chemical reactions release or absorb energy. The energy can be in the form of:
- thermal energy
- light
- sound
- electricity
Exergonic vs. Endergonic Reactions
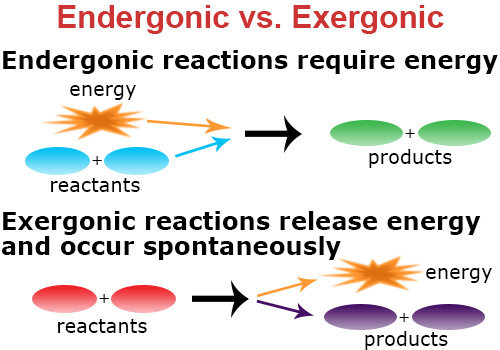
You are probably more familiar with those reactions that involve the release of energy. When a chemical reaction releases energy, it is known as an exergonic reaction.
Exergonic reactions:
- are spontaneous
- release energy into the surroundings
Examples of exergonic reactions include:
- chemiluminescence A firefly's abdomen produces light due to an exergonic chemical reaction, called chemiluminescence. It occurs when luciferase in the insect's lower abdomen reacts with oxygen to produce flashes of light (the energy that's released).
- cellular respiration During respiration, glucose (a sugar) and oxygen are converted to water and carbon dioxide, which are smaller molecules. This reaction releases a lot of energy, making it an exergonic reaction.

Fireflies use their abdomen lights as part of their courtship process.
An endergonic reaction is a chemical reaction that requires more energy to cause the reaction than energy that is released by the reaction.
These reactions:
- are not spontaneous
- require more energy than is returned from the reaction
- absorb energy from the surroundings so the reaction can occur
Examples of endergonic reactions include:
- photosynthesis Plants rely on the process of photosynthesis to survive. Photosynthesis requires a lot of energy, which plants get from the sun. Because this process requires added energy to occur, it is an endergonic reaction.
- melting ice into water Melting ice into water requires thermal energy, which is drawn from the substance's surroundings. Since this process requires energy to begin, it is an endergonic reaction.
Watch the video Energy Flow (2:26) to learn more. Login instructions.
Exothermic vs. Endothermic Chemical Reactions
An exothermic reaction occurs when energy is given off primarily in the form of thermal energy.
Examples of exothermic reactions include:
- fireworks
- burning fossil fuels
- hand and toe warmers
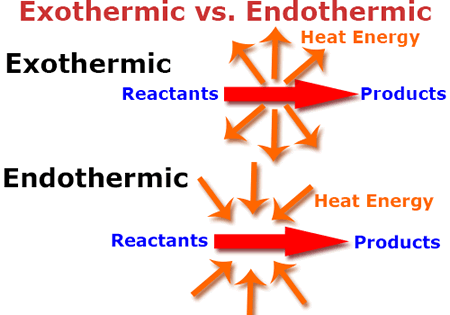
An endothermic reaction requires thermal energy to occur and to keep a reaction going.
Examples of endothermic reactions include:
- cooking
- photosynthesis
- cool packs
Watch the following videos to learn more. Login instructions.
- Energy and Chemical Reactions (1:35)
- Endothermic and Exothermic Changes (9:08)
- The Alan Nursall Experience Vol. 5: Purple Haze (2:44)
Review examples of exothermic and endothermic reactions below.
| Exothermic Processes | Endothermic Processes |
|---|---|
| Making ice cubes | Melting ice cubes |
| Formation of snow in the clouds | Conversion of frost to water vapor |
| Condensation of rain from water vapor | Evaporation of water |
| A candle flame | Forming a cation from an atom in the gas phase |
| Mixing sodium sulfite and bleach | Baking bread |
| Rusting iron | Cooking an egg |
| Burning sugar | Producing sugar by photosynthesis |
| Forming ion pairs | Separating ion pairs |
| Combining atoms to make a molecule in the gas phase | Splitting a gas molecule apart |
| Mixing water and strong acids | Mixing water and ammonium nitrate |
| Mixing water with an anhydrous salt | Making an anhydrous salt from a hydrate |
| Crystallizing liquid salts (as in sodium acetate in chemical handwarmers) | Melting solid salts |
Classifying Reactions
Now that we have defined different chemical reactions based on energy loss or gain, let's discuss how these classifications are related!
An easy way to differentiate between the two sets of classifications is:
- Endergonic and exergonic reactions describe reactions that occur with any form of energy.
- Endothermic and exothermic reactions only describe reactions that involve heat or thermal energy.
A chemical reaction can be both exothermic and exergonic, just as a chemical reaction can be both endothermic and endergonic. You can also say:
- All exothermic reactions are exergonic reactions.
- However, not all exergonic reactions are exothermic.
If a reaction involves the release of energy and that energy is emitted as thermal energy or heat, it can be described as both exergonic and exothermic.
If a reaction involves the release of energy but the energy is NOT released as heat, it can only be described as exergonic.
Similarly:
- All endothermic reactions are endergonic.
- However, not all endergonic reactions are endothermic.
If a reaction requires the addition of energy to occur and the added energy is thermal energy or heat, it can be described as both endergonic and endothermic.
If a reaction requires the addition of energy to occur and the added energy is NOT thermal energy or heat, it can only be described as endergonic.

