Learn
States of Matter
We know that matter is everything that has mass and takes up space. It is made of atoms and molecules, and in the previous lesson, we learned about different types of matter. Matter also has different states.
Take a look at the video States of Matter (3:03) to learn more. Login information.
Note: The presentation may take a moment to load.
Phase Changes
Because matter is based on the physical state of its atoms and molecules, they can change from one form to another. This is called a change of state or phase change. A solid can be heated to become a liquid, and a liquid can be heated to become a gas. We can see the change from solid to liquid, but we cannot always see the liquid change to a gas.
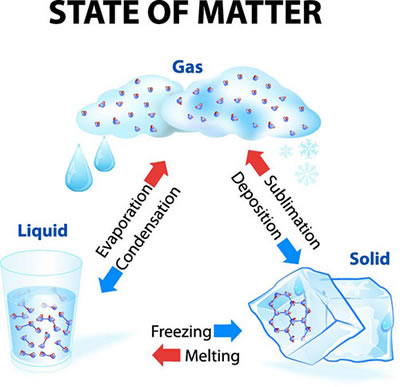
Matter can change state through processes like evaporation, condensation, sublimation, deposition, freezing, and melting.
Even though matter changes physical states, its chemical state remains stable and does not change. A good example of matter changing physical states but keeping its chemical state is water. Water can be a liquid and freeze to form a solid (ice); ice can melt back to liquid water, and both can evaporate to become a gas.
As temperature increases, a gas can ionize to become plasma. Conversely, as temperature decreases plasma can undergo recombination, also called deionization, to become a gas.
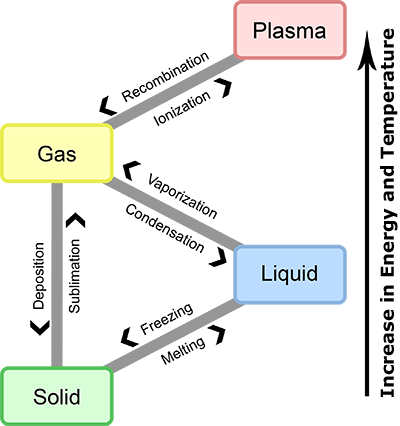
Temperature affects state of matter.
Next, review both the Particulate Nature of Matter Tutorial and Problem Set to learn more.
Energy Levels
States of matter can be categorized by energy level. In order from low energy to high energy:
- BE Condensates (lowest energy)
- solids
- liquids
- gases
- plasma (highest energy)
The more energy, the more active the atoms and molecules become.
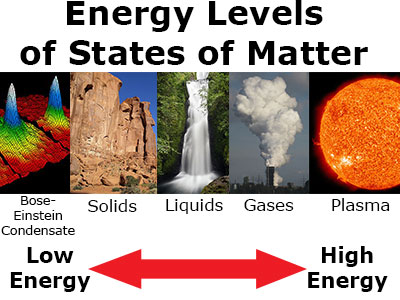
Energy levels of states of matter vary, with Bose-Einstein condensate having the lowest energy level and plasma having the highest energy level. Bose-Einstein image courtesy of the National Institute of Standards and Technology.
Let's say you make a dish of brownies. You put all the ingredients in the bowl, mix it up, and insert into the oven. Initially, you are the only person who can smell the brownies. As the oven continues to heat up and the brownies get hotter, the molecules in the brownies get more excited and and some molecules evaporate into a gas and escape the oven. Just before they are ready to come out of the oven, everyone in your house comes looking for brownies. Because a gas is confined only by its container, the excited gas that escaped from the oven has moved all around your house, filling it with the smell of brownies.

Temperature Changes
Most objects/substances expand as they heat and contract as they cool. Recall, all matter is made of atoms. In a solid, the atoms vibrate next to each other, atoms in a liquid are jostling around, and atoms in a gas are whizzing past each other. As heat is introduced, the atoms begin to move around more due to the increase of kinetic energy.
Watch the video Thermal Expansion of Solids (2:11) to learn how solids react to temperature changes. Login instructions.

To account for thermal expansion, expansion joints like the one above are included on bridges and highways.
Water is the exception to this rule of thermal expansion. Watch Anomalous Expansion of Water (4:55) to see the types of bonds that are formed by water molecules during the freezing phase. Login instructions.

