Learn
Detecting Poison
Spectroscopy
Spectroscopy involves shining light on objects to see how they respond.
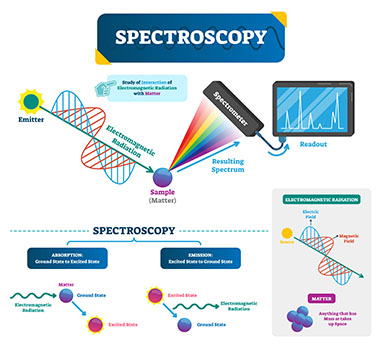
Spectroscopy and Electromagnetic Radiation
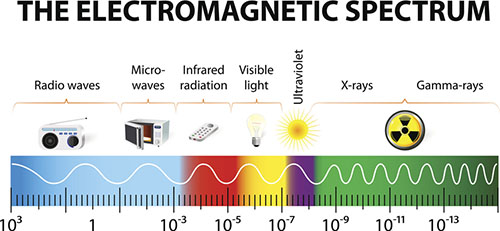
There are various kinds of spectroscopy that utilize different types of electromagnetic radiation to study the characteristics of matter.
- For example, radio waves are used to determine the arrangement of carbon and hydrogen atoms in nuclear magnetic resonance (NMR) spectroscopy.
- Ultraviolet (UV) and visible light are used to determine the types of bonds present in a compound.
In forensics, spectroscopy is applied in analyzing gunpowder, fibers, paint samples, and glass samples. Spectroscopy is advantageous because it is non-destructive and requires little time and sample preparation.
Raman Spectroscopy
In the field of forensic toxicology, Raman spectroscopy is commonly used to confirm the presence of certain drugs or determine the amount of drug contained in a sample. This method examines how a sample scatters light due to vibrations in the molecules.

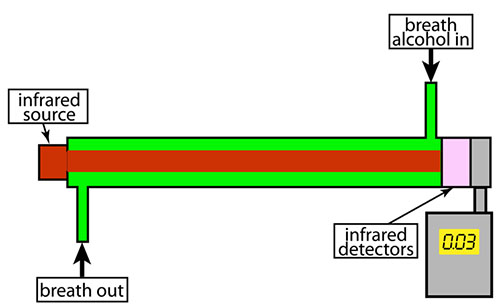
Breathalyzers and Infrared Radiation
Some breathalyzers use infrared radiation (IR) to determine the amount of ethanol in a sample. Ethanol, which is also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol, will absorb specific wavelengths of IR and the amount of IR absorbed at that wavelength can indicate the amount of ethanol present.
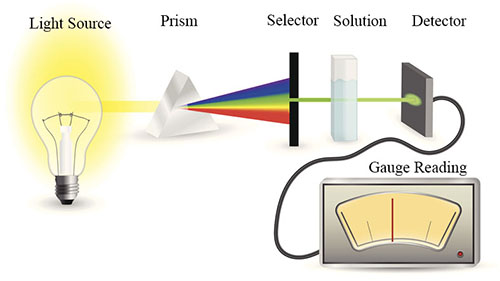
UV-Visible Spectrophotometry
We will focus on a simple version of spectroscopy - UV-Visible spectrophotometry. In this method, various colors of light are shone on a sample to determine which wavelength of light is best absorbed by the sample. The wavelength of light absorbed provides information about the contents of the sample.
For example, this sample is absorbing the most light at around 620 nm.

How UV-Visible Spectrometry Works
Once the color of light has been determined, the amount of light absorbed can be measured and used to determine the concentration of the sample.
Calibration Curve Graphs
Samples with known concentration are used to create a calibration curve, as seen below. Using this graph, the concentration of an unknown could be determined based on its absorbance.
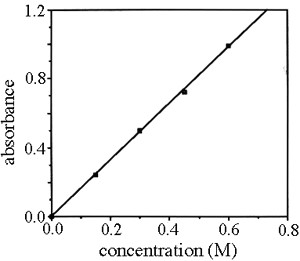
Knowledge Check #1
How many known samples were used to create this calibration curve?

- 2
- 3
- 5
- The number cannot be determined from the graph.
Answer: c. 5
Knowledge Check #2
What is the approximate absorbance of a sample with concentration of 0.3 M?

- 2
- 0.5
- 0.8
- 1.2
Answer: b. 0.5
Knowledge Check #3
If an unknown sample had an absorbance of 0.7, what would the approximate concentration be?

- 0.21 M
- 0.32 M
- 0.43 M
- 1.27 M
Answer: c. 0.43 M