Learn
The Science of Arson
Arson
Utilization of fire is essential to our survival. However, fire is also one of the most destructive natural forces, with the power to reduce buildings or forests to ash in just a few hours. While some fires are caused by accidents or natural forces, fires may also be set intentionally—a crime known as arson. Due to the somewhat unpredictable nature of fire, arson investigation requires experts with extensive training.

Chemistry of Fire
Before we discuss arson cases, what are the components needed for a fire to exist? Fire is both a physical and chemical process. For a fire to occur three things are required:
- A Fuel Source - Some sort of flammable substance must be present.
- Oxygen - Enough oxygen must be present to sustain the combustion. Oxygen makes up about 20% of the air we breathe.
- Heat - Enough heat must be present to ignite the fuel source. The minimum temperature required to ignite a liquid fuel source is called flash point.
Fire Triangle
These three components - fuel, oxygen, and heat - can be illustrated by the fire triangle.

For a fire to start, all three of these components within the fire triangle must be present. If one of these components is removed, the fire will go out.
Knowledge Check #1
What are the three things required for a fire to occur?
- Heat, Humans, and Error
- Fuel, Oxygen, and Heat
- Humans, Matches, Fuel
Answer: b. Fuel, Oxygen, and Heat
Knowledge Check #2
There are three things required for a fire to occur. What happens if one of those things is removed?
- The fire will get hotter.
- The fire will get less intense.
- The fire will go out.
Answer: c. The fire will go out.
Knowledge Check #3
What percentage of the atmosphere is oxygen?
- 10%
- 20%
- 40%
- 60%
Answer: b. 20%
Chemical Reaction
Fire is a chemical reaction, which is when molecules are broken down and rearranged as they combine to produce new substances. More specifically, burning is a type of reaction known as combustion, or the rapid combination of a substance with oxygen, accompanied by heat and light.
Chemical Equation for Combustion
Most fuel sources contain carbon and hydrogen in some ratio (CxHy), which combines with oxygen to form carbon dioxide (CO2) and water (H2O). A simplified chemical equation for combustion may be expressed as:
Fuel (CxHy) + oxygen (02) + spark → water (H2O) + carbon dioxide (CO2) + heat
The substances on the left side of the arrow are known as the reactants or starting materials. The substances on the right side of the arrow are the products.
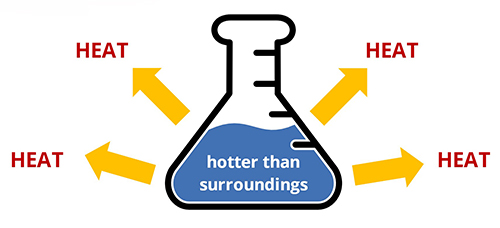
Exothermic
Because heat is a product of combustion reactions, combustion reactions are exothermic, meaning that they are processes that release heat.
Knowledge Check #4
Water is a _________ in combustion reactions.
- product
- reactant
Answer: a. product
Knowledge Check #5
Due to the high temperatures typically present in a combustion reaction, water is usually produced as a ____________, known as water vapor.
- solid
- liquid
- gas
Answer: c. gas
What Starts a Combustion Reaction?
For a combustion reaction to occur, the reactants (or fuel) must reach a certain temperature. The temperature required varies depending on:
- The Fuel Source - Some materials ignite at lower temperatures and are more prone to causing fires.
- The Presence or Absence of a Spark or Ignition Source - Without a spark or ignition source, very high temperatures are typically required for a fire to start. This temperature is known as the auto-ignition temperature.
- For wood, the auto-ignition temperature is 572°F
Flash Point
In the presence of a spark or ignition source, liquid fuels will ignite at lower temperatures called the flash point.
Flash points are typically much lower than auto-ignition temperatures. Materials with flash points below 140°F are considered flammable, while while those with flash points above 140°F are classified as combustible.

Flash Point and Auto-Ignition of Fuels
Examine the flash point and auto-ignition temperatures of common fuels.
| Fuel | Flash Point (°F) | Auto-Ignition Temperature (°F) |
|---|---|---|
| Ethanol | 61.9 | 685 |
| Gasoline | -45 | 536 |
| Diesel | 126 | 493 |
| Jet Fuel | 100 | 410 |
| Kerosene | 100-162 | 428 |
| Vegetable Oil | 621 | 795 |
Knowledge Check #6
Which substances from the table above would be considered flammable?
- ethanol
- ethanol, gasoline, jet fuel
- ethanol, gasoline, diesel, jet fuel, kerosene
- All of the fuel sources on the chart are flammable.
Answer: c. ethanol, gasoline, diesel, jet fuel, kerosene
Knowledge Check #7
It is sunny spring day with a temperature of 65°F. Which of these substances from the table above would ignite if a spark, such as from a match, was present?
- ethanol and gasoline
- diesel, jet fuel, and kerosene
- All these substances will ignite if a spark is present.
Answer: a. ethanol and gasoline
Knowledge Check #8
True or False: Flash points are typically higher than auto-ignition temperatures.
- True
- False
Answer: b. False
Types of Combustion
Depending on the availability of oxygen, combustion may be complete or incomplete.
- In complete combustion, oxygen is plentiful, and the fuel source reacts completely, producing primarily carbon dioxide and water. The burning of fuel in your car is one example of complete combustion.
- Incomplete combustion takes place when there is not enough oxygen present to complete the reaction. In this case, instead of carbon dioxide being produced, carbon and carbon monoxide an odorless, colorless, poisonous gas are also produced, contaminating the smoke.
Complete and Incomplete Combustion
Open Complete & Incomplete Combustion in a new window
Note: The presentation may take a moment to load.
Knowledge Check #9
Do the following statements best describe complete or incomplete combustion? Match the statements to each category:
Categories:
- Complete Combustion
- Incomplete Combustion
Statements:
- Products are toxic and pollutants
- Plentiful supply of air
- Produces carbon dioxide and water
- Limited supply of air
- Produces carbon or carbon monoxide and water
- Product contributes to global warming
Answer:
- Complete Combustion:
- Product contributes to global warming
- Plentiful supply of air
- Produces carbon dioxide and water
- Incomplete Combustion:
- Produces carbon or carbon monoxide and water
- Limited supply of air
- Products are toxic and pollutants
Combustion Reactions
Combustion reactions can also be classified according to how they began and the speed at which they propagate spread .
- Smoldering - A slow, flameless, low-temperature combustion of a solid fuel, such as wood, coal, or cotton. Smoldering is a very localized burn that produced thick, tarry smoke. Smoldering fires can turn into flames if enough oxygen is present.

Smoldering charcoal is an example of a flameless combustion reaction. - Rapid Combustion - Gas burns quickly, producing heat and light in the form of a flame. This is the type of combustion we most associate with fires.

Turning on a gas stove ignites the burner quickly, so it's an example of rapid combustion. 
Striking a match to light it is another example of rapid combustion. - Spontaneous Combustion - Materials burst into flames without the application of a spark. This generally occurs because of reactions occurring within the material that release sufficient heat to cause ignition (the auto-ignition temperature).

Forest fires are examples of spontaneous combustion, as the dry grasses and leaves start burning in the summer heat. Other examples are spontaneous fires that start in coal mines when the coal dust interacts with oxygen in the air. - Explosive Combustion - A very fast combustion reaction in which a large of amount of gaseous product is quickly produced, causing rapid expansion and a loud sound.

Fireworks are examples of explosive combustion. Sparks light the fuse, exploding the firework and producing a lot of energy in the form of heat, light, and sound.
Knowledge Check #10
Is it possible for a person to spontaneously combust? There have been several cases throughout history where a person seemed to burn from the inside out. Open Know about the facts and theories of spontaneous human combustion and either watch the video or read the transcript.
True or False: Matilda Rooney spontaneously combust.
- True
- False
Answer: b. False
Knowledge Check #11
Match the following examples to the type of combustion reaction:
Types of Combustion Reactions:
- Rapid Combustion
- Smoldering
- Explosive Combustion
- Spontaneous Combustion
Examples:
- a cigarette butt is left on a couch cushion
- a candle burning
- an oil-soaked rag suddenly ignites
- fireworks
Answers:
- Rapid Combustion - a candle burning
- Smoldering - a cigarette butt is left on a couch cushion
- Explosive Combustion - fireworks
- Spontaneous Combustion - an oil-soaked rag suddenly ignites