Learn

Formation
Ozone is found in earth's atmosphere. It is typically located in the stratosphere layer of the atmosphere.
 Ozone is made up of a covalent bond in which electrons are shared between 3 oxygen atoms and has the chemical formula (O3).
Ozone is made up of a covalent bond in which electrons are shared between 3 oxygen atoms and has the chemical formula (O3).
Atmospheric oxygen is a diatomic molecule — two atoms of oxygen joined together to form a molecule (O2).
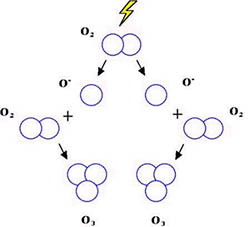
Ozone is formed naturally in the stratosphere when atmospheric oxygen (O2) in the stratosphere is broken apart in a chemical reaction by ultraviolet radiation from the sun producing 2 oxygen atoms. Next these free oxygen atoms combine with an atmospheric oxygen molecule (O2) to make ozone (O3). In this reaction, 3 oxygen molecules (O2) produce 3 ozone molecules (O3). Ozone is continually being formed in the earth's stratosphere through this process.

Importance
Ozone is a very rare molecule in our atmosphere. There are about 3 molecules of ozone for every 10 million air molecule. Even though ozone occurs in such small amounts, its presence in the stratosphere plays an important role in making life on earth possible.
Stratospheric ozone is often referred to as "good ozone". It is responsible for absorbing most of the ultraviolet sunlight that is damaging to organisms. Without the ozone in the stratosphere, more of the UV radiation from the sun would reach the earth. Ultraviolet radiation can cause, sunburn, skin cancer, premature skin aging, cataracts/eye damage, and immune system suppression.
Not all of the UV radiation is absorbed by the ozone. This is good, because some UV radiation is beneficial for people. It is responsible for the production of vitamin D. It can also be used to treat diseases such as psoriasis, eczema, and jaundice.

Destruction
Ozone is destroyed when CFCs
chlorofluorocarbons
and other ozone depleting substances
chlorofluorocarbons
(ODS)—like hydrochlorofluorocarbons
HCFCs
, halons
used in fire extinguishers
, methyl bromide
used in pesticides
, methyl chloroform
used as a solvent in industrial processes
— are released into the atmosphere. These ozone-depleting substances enter the troposphere where they are evenly distributed and can stay for years because they are extremely stable and do not dissolve in rain. After several years, these molecules enter the stratosphere where they are broken down by UV light. The byproducts when the substances are broken down are what contribute to ozone depletion in the atmosphere.
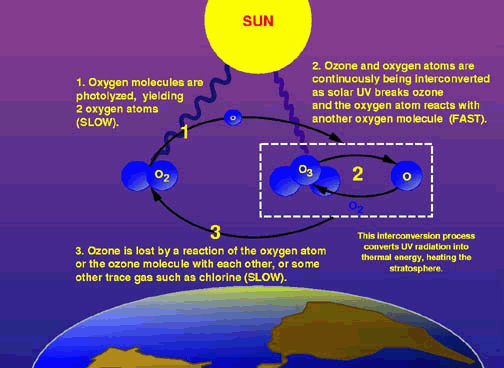
The diagram above shows the general process leading to the breakdown of O3. While O2 and O3 are naturally interconverted, the slower process of losing ozone molecules when they react with a trace gas like chlorine permanently removes O3 molecules from the cycle, gradually reducing the amounts of O3 available in the atmospehere.
When CFCs are broken down, chlorine atoms and halons compounds consisting of carbon, bromine, and fluorine are formed. These are the components are what actually destroy ozone. It is estimated that one chlorine molecule can breakdown 100,000 ozone molecules. As methyl bromides and halons are broken apart, the release bromine atoms which are 60 times more destructive to ozone molecules than chlorine. Visit EPA: Ozone-depleting Substances to see the different classes of ozone depleting substances. Visit EPA: Health and Environmental Effects of Ozone Layer Depletion.
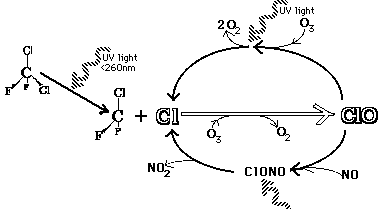
The diagram above shows the chemical process leading to the breakdown of O3. CFCs react with UV light and release a chlorine ion. That chlorine ion "pulls" an oxygen atom from O3, resulting in the formatino of chlorine oxide, ClO, and changing the O3 into O2.

History
CFCs were first manufactured in the 1930s. They quickly became beneficial in industry because of their stability. CFCs are commonly used in refrigerants for air conditioners and refrigerators, solvents, and blowing agents. CFCs used to be components used in aerosol cans as a propellant for the content within the bottle — ex. hairspray. CFCs were banned as propellants in hairspray cans in 1978.
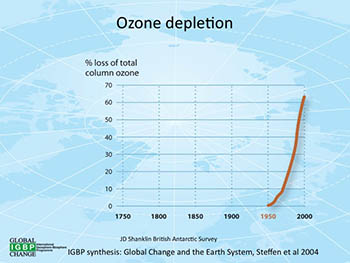
This graphic shows how the rate of ozone loss has skyrocketed from 1950 through 2000. Click on the graph for a larger version.
In the 1980s, scientists discovered a hole in the ozone over Antarctica which was thought to be caused by CFC emissions into the atmosphere. This discovery led to the Montreal Protocol in 1987 being signed to reduce the use of CFCs and other ozone depleting substances. CFC production was officially banned except for in essential uses. HCFCs compounds began being used to replace CFCs. They are not as harmful to the ozone although they do deplete the ozone some. The United States was one of the countries signing the Montreal Protocol. As a result, they have agreed to phase out HCFC consumption and production with a complete HCFC phase out by 2030.
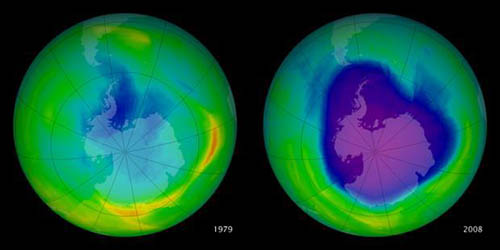
This graphic shows the hole in the ozone layer over Antarctica as it has grown from 1979-2008. The scale moves from larger amounts of ozone on the red end of the spectrum to lower amounts of ozone as the color shifts towards purple.
In 1993, the Clean Air Act passed in 1970 by congress and amended several times: requires the Environmental Protection Agency [EPA] to develop and enforce regulations to protect the public from airborne contaminants that can be harmful to human health imposed a phaseout framework to reduce the most harmful HCFCs being used. This helps meet the mandate set in the Montreal Protocol.

Troposphere
Very low concentrations of ozone occur naturally in the troposphere from hydrocarbons that are released by plants and the soil, as well as, some ozone molecules that migrate down from the stratosphere. However, these levels of ozone are not significant enough to cause problems to the ecosystem or humans.
The ozone that is known as "bad ozone" in the troposphere is not assembled in the troposphere the same way it is in the stratosphere. It is a result of pollutants, such as nitrogen oxides, carbon monoxides, and volatile organic compounds VOCs are carbon based compounds such as acetone, benzene, and xylene that evaporate easily. They are found in consumer products such as paint, carpets, adhesives, and upholstery fabric. , that are released into the atmosphere during the burning of fossil fuels. These compounds react in the atmosphere in the presence of sunlight creating ozone. These reactions that create ozone are known as photochemical reactions — chemical reactions occurring in the presence of sunlight.
This type of ozone is considered a pollutant. Since 1900, the amount of ozone in the troposphere has nearly doubled. This is a result of more automobiles and factories that burn fossil fuels. Increased levels of ozone can cause damage to lung tissue, creating more breathing difficulties for people with respiratory problems like asthma. Ozone also can cause eye irritation, and lead to health problems such as heart disease, bronchitis, and emphysema. You may have notice on weather reports where they have issued reports on ozone levels and encourage individuals with respiratory problems to stay inside. When mixtures of pollutants from the combustion of fossil fuels and ozone combine in the troposphere, it is usually referred to as photochemical smog, also known as just plain smog.
Ozone levels in the troposphere can be reduced by decreasing the use of fossil fuels. Things like using public transportation and carpooling are ways that individuals can help reduce ozone levels and pollution to the earth's troposphere.
 |
 |