Learn

Carbon Compounds
Carbon compounds are found in all areas of the environment from carbon dioxide in the atmosphere to carbon compounds in rocks and soil. Living organisms are often referred to as carbon-based life forms because the same carbon atoms that are found in compounds in the environment also make up the core structures for molecules within living organisms.

Carbon is essential to your DNA
Although living organisms are mostly water, carbon combines with hydrogen, oxygen, nitrogen, and phosphorus atoms to form the majority of the compounds found in living organisms. Carbon can form long chains called polymers to form important biological molecules, such as proteins, lipids fatty acids , DNA and RNA nucleic acids , and carbohydrates sugars = food energy .
- Proteins are the building blocks for all living things. They make up enzymes that tell the cell what to do by controlling all biological processes.
- Lipids contribute to the makeup of cell membranes, store energy, and insulate against cold.
- Nucleic acids store the genetic information for life. DNA has genes that are responsible for making the proteins that control cellular functions. RNA is responsible for assembling these proteins.
- Carbohydrates are responsible for most of the energy that our bodies store and use to do work.
All of these macromolecules
very large molecule
are important to supporting life functions and/or allowing life to reproduce.

Carbon Cycle
Carbon based compounds are the basis for organic chemistry chemistry involving the carbon based compounds that are important to life . Because of its importance to all life, it is vital that carbon is distributed throughout the environment.
Much like water molecules, carbon atoms cycle through the environment between living organisms, the atmosphere, and the soil. Because living things are made of carbon based compounds, carbon is
- transferred when animals eat plants and other animals;
- transferred to the atmosphere in the form of carbon dioxide (CO2) when organisms die and breakdown;
- introduced into the soil when organisms die and are fossilized in rocks.
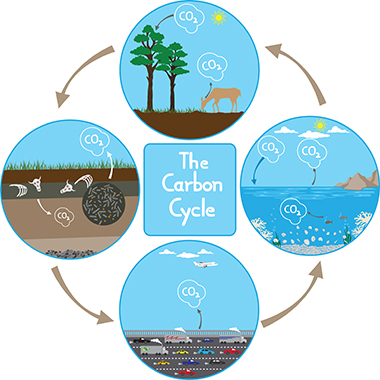
When organic material, such as fossils or plant and animal remains is burned, CO2 is released into the atmosphere. The burning of organic materials, especially fossils, is known as combustion.
Plants take in CO2 to use in photosynthesis. During photosynthesis, plants convert CO2 into sugar, the carbon compound that is used as food energy.
When humans carry out respiration, which is the breaking down of sugar to release energy to carry out biological processes, CO2 is exhaled into the environment.
Aquatic
water
plants take in CO2 that is present in the water and use it in photosynthesis just like terrestrial
land
plants. There is a continual exchange of CO2 between the atmosphere and the oceans. When CO2 enters the ocean, it forms carbonic acid and bicarbonate. This allows the ocean to absorb a large amount of CO2. The shells of may aquatic organisms, such as oysters, clams, and snails are formed from a carbon compound called calcium carbonate.

Oxygen
Oxygen is tied to the carbon cycle in the processes of photosynthesis and respiration.
-
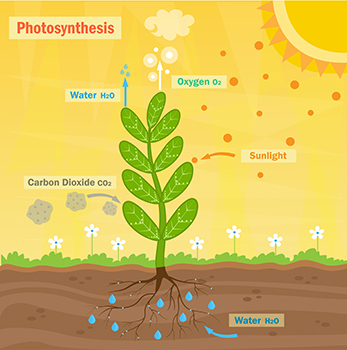
In photosynthesis, carbon dioxide is taken in by plants to make food and oxygen. Oxygen is a byproduct of photosynthesis, and it is released into the atmosphere. The chemical equation for photosynthesis is:
6CO2 + 6H2O + light energy → C6H12O6 + 602
-
In respiration, oxygen is taken in and carbon dioxide is released into the atmosphere. Carbon dioxide is a byproduct of respiration. Aquatic animals take in the oxygen that is dissolved in the water and use it in cellular respiration just like terrestrial animals. The chemical equation for respiration is:
C6H12O6 + 602 → 6CO2 + 6H2O + energy
Both of the equations above represent the carbon and oxygen cycle.
![]() Go to Science Learning: Carbon Cycle to explore more about the carbon and oxygen cycle. Click on the different labels to view short video clips or images about different parts of the cycle.
Go to Science Learning: Carbon Cycle to explore more about the carbon and oxygen cycle. Click on the different labels to view short video clips or images about different parts of the cycle.
 |
 |