Learn

Characteristics of Water
Water has many unique properties that make it essential for life.
Water is the only natural substance that can be found in all 3 physical phases / states — gas, liquid, and solid — at temperatures found on earth. Water boils at 100 degrees Celsius (212 degrees Fahrenheit) and begins to freeze at 0 degrees Celsius (32 degrees Fahrenheit).

Water shown in all 3 states, solid, liquid, and gas.

Polarity
Water is made of 2 atoms of hydrogen joined with 1 atom of oxygen in a covalent electron sharing bond. The electrons are shared between the hydrogen and oxygen atoms. Each of these bonds forms a molecule of water. Because the oxygen atom has a stronger pull on the negatively charged electrons, the electrons shared are actually a little closer to the oxygen molecule. This creates a polar molecule — as a somewhat positive end and a somewhat negative end — the oxygen end is more negative because the electrons are closer to it and the hydrogen end is more positive. This polarity gives the water molecule several properties that are important for life functions.
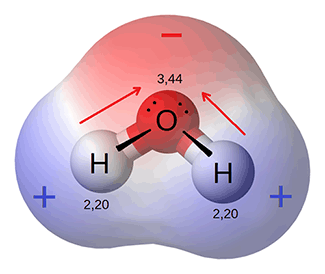
This diagram illustrates the polarity of water. As the electrons (black dots) are pulled more closely to the oxygen, that end of the molecule becomes more negative than the ends with the hydrogen atoms.

Cohesion
You have seen other words that have the prefix "co". Words such as cooperate, costar, coworker, coexistence — all meaning together, jointly, united, or cling. Because water molecules are polar, they have a property known as cohesion — water molecules are attracted to other water molecules. This property allows water molecules to "stick" together. The positive ends are attracted to the negative ends.

Cohesion is the property of water that creates surface tension. Water molecules at the top of water have a greater cohesive force because there are fewer of them. This creates water's surface tension. Water's surface tension is what allows small objects to float on top of the water. Surface tension is what allows small insects — such as water striders — to look as if they are walking on top of the water. As long as the insect or object does not break the surface tension, it will be able to "sit" on top of the water.

This water strider takes advantage of surface tension to "walk" on water.
Surface tension causes water to bead up on clothes and other surfaces. This inhibits the cleaning process. This is the reasons that detergents are added to water to help with cleaning. Detergents help reduce the surface tension for water to soak in and clean the fabric.

Adhesion
Capillary action takes place when the adhesive forces (2 different substances) are stronger than the cohesive forces (2 of the same substance). When the nurse pricks your finger for blood, they use a very narrow tube called a capillary tube. It is this capillary action that allows the blood to "travel" up this tube. Another important property of water that is created by its polarity is adhesion. Adhesion is the property of water molecules "sticking" to other substances.
The properties of cohesion and adhesion join forces to create capillary action. Capillary action is the process that allows water to plants to get the water and nutrients that they need. Water carries the nutrients that plants need and they enter the plant from the ground through its roots. The water molecules are sticking to each other and the roots and other parts of the plant. This allows the water to be soaked up and travel through the roots to the stem to the rest of the plant.

These drops of dew illustrate cohesion and adhesion. The water molecules are attracted to one another, forming the droplet, and "stick" to the plant, keeping the dropplet attached to the plant itself.
Capillary action can be seen when you use paper towels to clean up a water spill. The water molecules cohesive property causes the puddle of water on the floor after the spill. When you put a paper towel on the water, the water molecules "stick" to the paper towel by adhesion. These two properties allow the water molecules to travel into the paper towel.

Solution
Water's polarity—having a positive and negative end—allows it to dissolve a wide range of substances. It is attracted to different substances and is able to dissolve more substances than any other liquid. Water is able to break apart molecules that have positive and negative particles, thus dissolving them. For this reason, it is called the "Universal Solvent". This is an important property of water because nutrients enter plants and other organisms in water. This means that the nutrients have to be dissolved in water. Because it can dissolve a wide range of substances, it is able to dissolve the nutrients and transport them throughout the organism's body.
Pure water is not able to conduct electricity, but when water has dissolved substances such as NaCl (sodium chloride, salt) and other ions (when substances dissolve in water, atoms of the compound are broken down into positive and negative atoms called ions), it has the ability to conduct electricity. This is important because many organisms, such as humans, require electrical impulses to be sent from the brain to the rest of the body (and from the body to the brain). Without these dissolved substances, this would not be possible.

Heat Capacity
Another important property of water is its ability to absorb heat and be a coolant. Water is a good coolant because it has a high specific heat capacity. This means that water is able to absorb a large amount of heat without becoming hot. It takes more heat to raise the temperature of 1 gram of water by 1 degree Celsius than it takes to raise the temperature of 1 gram of copper by the same amount.
This unique property of water makes it valuable to industry as a coolant. In factories there are often large machines that have moving parts. When these moving parts rub together there is friction which creates heat. These machines need to be cooled in order to keep them running. Water is cycled through them to absorb the heat and protect the machine.

These cooling towers take advantage of water's high specific heat capacity to absorb heat.
If you have ever been to the beach, lake, or in a swimming pool, you have seen the effects of water's specific heat. Once the water absorbs the heat during the day, it will retain it longer than the solids on the beach. So, in the morning, the solids and the air have lost their heat (becoming cooler) and the water will then feel relatively warmer, since it has retained much of the heat from the day before.
Not only does it take water longer to heat up than most other substances, it also takes water longer to cool down. This is also related to its specific heat capacity. The property of water taking longer to heat up and cool down is important to the environment. It helps insure that there are not dramatic, extreme, sudden changes in water temperature throughout the day and over time. Areas next to large bodies of water often have a more moderate climate because water heats up slow and cools down slow.
 |
 |